
… I propose to call the magnitude S the entropy of the body… I have intentionally formed the word entropy so as to be as similar as possible to the word energy…. In the second place, with each non-reversible change is associated an uncompensated transformation…
… the quantities of heat which must be imparted to, or withdrawn from a changeable body are not the same, when these changes occur in a non-reversible manner, as they are when the same changes occur reversibly. In The Mechanical Theory of Heat, Clausius explains his findings: Perplexed, scientists asked, where did the rest of the heat go and why?Ĭlausius solved the riddle by observing a steam engine and calculating that energy spread out and left the system. But if you want to heat cold water to make the coffee, you need to do work - you need a power source to heat the water.įrom this idea comes Clausius’s statement of the second law of thermodynamics: “heat does not pass from a body at low temperature to one at high temperature without an accompanying change elsewhere.”Ĭlausius also observed that heat-powered devices worked in an unexpected manner: Only a percentage of the energy was converted into actual work. This is how your coffee cools down the longer it’s left out - the heat from the coffee flows into the room. He recognized that heat from a body at a high temperature would flow to one at a lower temperature. Clausius was oblivious to Carnot’s work but hit on the same ideas.Ĭlausius studied the conversion of heat into work. I say attributed because it was a young French engineer, Sadi Carnot (1796–1832), who first hit on the idea of thermodynamic efficiency however, the idea was so foreign to people at the time that it had little impact. The identification of entropy is attributed to Rudolf Clausius (1822–1888), a German mathematician and physicist. Let’s take a look at what entropy is, why it occurs, and whether or not we can prevent it. Zoom out a lot further, and we see the entire universe marching towards a collapse. Zoom out a little, and businesses are failing, crimes and revolutions are occurring, and relationships are ending. Cells within your body are dying and degrading, an employee or coworker is making a mistake, the floor is getting dusty, and the heat from your coffee is spreading out. Thy hand, great Anarch! lets the curtain fall Īnd universal darkness buries all.” - Alexander Pope, The DunciadĪs you read this article, entropy is all around you. Lo! thy dread empire, Chaos! is restored Nor human spark is left, nor glimpse divine! “Nor public flame, nor private, dares to shine
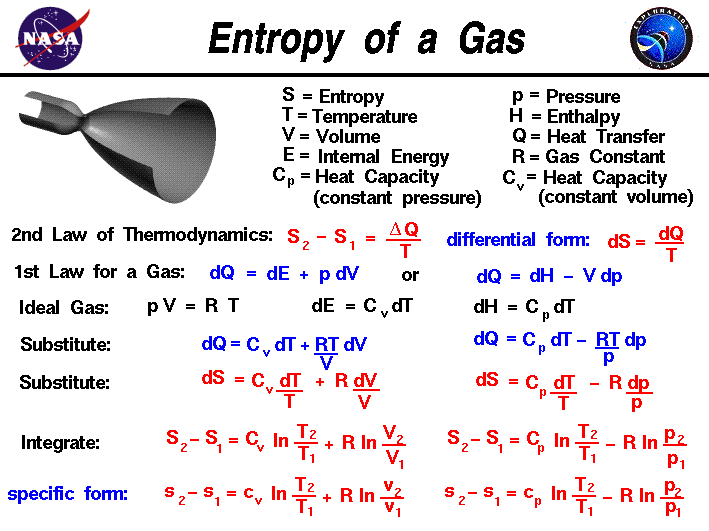
The Greek root of the word translates to “a turning towards transformation” - with that transformation being chaos. In short, we can define entropy as a measure of the disorder of the universe, on both a macro and a microscopic level. The more disordered something is, the more entropic we consider it. Energy disperses, and systems dissolve into chaos. Left unchecked disorder increases over time. In fact, you can think of it as nature’s tax. More specifically, the second law of thermodynamics states that “as one goes forward in time, the net entropy (degree of disorder) of any isolated or closed system will always increase (or at least stay the same).” Įntropy is simply a measure of disorder and affects all aspects of our daily lives. Entropy, a measure of disorder, explains why life seems to get more, not less, complicated as time goes on.Īll things trend toward disorder.


 0 kommentar(er)
0 kommentar(er)
